Seznamy Atom Number Čerstvý
Seznamy Atom Number Čerstvý. More commonly, the isotope symbol already tells you the atomic number. The atomic number is the smaller of the two numbers in the symbol. The atomic number density (n; The mass number of an atom is its total number of protons and neutrons.
Prezentováno Atomic Number And Mass Number Introduction Notation Examples
Atomic number (z) = no of protons (p + ) Atoms/cm 3) is the number of atoms of a given type per unit volume (v; More commonly, the isotope symbol already tells you the atomic number.20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.
Mass number = number of protons + … Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; The atomic number density (n; The atomic number is the smaller of the two numbers in the symbol.
Atomic number is the number of protons there in an atom. 19.04.2016 · the atomic number of carbon is 6. Mathematically, the atomic number is defined as.

Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. Atomic number (z) = no of protons (p + ) Cm 3) of the material. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. (3.4.1) number of neutrons = rounded mass number − atomic number. For example, if the symbol is written as 146 c, the number 6 is listed. The atomic number density (n; Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; Atoms of different elements usually have different mass numbers , but they can be the same.

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … .. 19.04.2016 · the atomic number of carbon is 6.

The atomic number is the smaller of the two numbers in the symbol. .. Atoms/cm 3) is the number of atoms of a given type per unit volume (v;

20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. More commonly, the isotope symbol already tells you the atomic number. Mathematically, the atomic number is defined as. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Mass number = number of protons + … The mass number of an atom is its total number of protons and neutrons. Atoms/cm 3 ) of a pure material having atomic or molecular weight (m;. Atoms/cm 3 ) of a pure material having atomic or molecular weight (m;

Mathematically, the atomic number is defined as.. Atoms of different elements usually have different mass numbers , but they can be the same. 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Atoms/cm 3) is the number of atoms of a given type per unit volume (v; 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.

Atoms/cm 3) is the number of atoms of a given type per unit volume (v; Cm 3) of the material. Atomic number is the number of protons there in an atom. Mathematically, the atomic number is defined as... (3.4.1) number of neutrons = rounded mass number − atomic number.

For example, if the symbol is written as 146 c, the number 6 is listed... Grams/mol) and the material density (⍴; Mathematically, the atomic number is defined as. Mass number = number of protons + … Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … On the other hand, the number of neutrons for a given element can differ.

Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. Atoms of different elements usually have different mass numbers , but they can be the same. The atomic number density (n; On the other hand, the number of neutrons for a given element can differ. The atomic number is the smaller of the two numbers in the symbol. 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. Grams/mol) and the material density (⍴; (3.4.1) number of neutrons = rounded mass number − atomic number. Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … Together, the number of protons and the number of neutrons dictates an element's mass number. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the …. The atomic number is the smaller of the two numbers in the symbol.

Atoms/cm 3) is the number of atoms of a given type per unit volume (v; Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. On the other hand, the number of neutrons for a given element can differ. Cm 3) of the material. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … Atoms/cm 3) is the number of atoms of a given type per unit volume (v; The atomic number density (n; 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.. Grams/mol) and the material density (⍴;

Atomic number is the number of protons there in an atom.. (3.4.1) number of neutrons = rounded mass number − atomic number. Atomic number is the number of protons there in an atom. The atomic number density (n; On the other hand, the number of neutrons for a given element can differ. It is typically located as a subscript to the left of the element symbol. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Atomic number (z) = no of protons (p + ) 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). 19.04.2016 · the atomic number of carbon is 6. The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. Atomic number is the number of protons there in an atom. On the other hand, the number of neutrons for a given element can differ. Grams/mol) and the material density (⍴; Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. 17.01.2021 · in atoms, there are a total of four quantum numbers: Atomic number (z) = no of protons (p + ). More commonly, the isotope symbol already tells you the atomic number.

It is typically located as a subscript to the left of the element symbol. More commonly, the isotope symbol already tells you the atomic number. 19.04.2016 · the atomic number of carbon is 6. The atomic number density (n; 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.. Together, the number of protons and the number of neutrons dictates an element's mass number.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
It is typically located as a subscript to the left of the element symbol. Grams/mol) and the material density (⍴; The atomic number density (n; Atoms/cm 3) is the number of atoms of a given type per unit volume (v;

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Atoms of different elements usually have different mass numbers , but they can be the same.. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).

Together, the number of protons and the number of neutrons dictates an element's mass number.. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … Atomic number (z) = no of protons (p + ) Atoms of different elements usually have different mass numbers , but they can be the same. For example, if the symbol is written as 146 c, the number 6 is listed.. Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 …

More commonly, the isotope symbol already tells you the atomic number. The atomic number density (n; Grams/mol) and the material density (⍴; Mathematically, the atomic number is defined as. Atomic number is the number of protons there in an atom. 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … Together, the number of protons and the number of neutrons dictates an element's mass number.. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52.

More commonly, the isotope symbol already tells you the atomic number. The atomic number density (n; Atomic number is the number of protons there in an atom. Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; The mass number of an atom is its total number of protons and neutrons. The atomic number is the smaller of the two numbers in the symbol. Cm 3) of the material. Mass number = number of protons + … 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Together, the number of protons and the number of neutrons dictates an element's mass number. (3.4.1) number of neutrons = rounded mass number − atomic number.. The mass number of an atom is its total number of protons and neutrons.

More commonly, the isotope symbol already tells you the atomic number.. It is typically located as a subscript to the left of the element symbol. Atomic number is the number of protons there in an atom. Mass number = number of protons + … 17.01.2021 · in atoms, there are a total of four quantum numbers: The mass number of an atom is its total number of protons and neutrons. 19.04.2016 · the atomic number of carbon is 6. For example, if the symbol is written as 146 c, the number 6 is listed. Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. Together, the number of protons and the number of neutrons dictates an element's mass number.

19.04.2016 · the atomic number of carbon is 6. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). 19.04.2016 · the atomic number of carbon is 6. The atomic number is the smaller of the two numbers in the symbol. On the other hand, the number of neutrons for a given element can differ. Cm 3) of the material. The mass number of an atom is its total number of protons and neutrons. For example, if the symbol is written as 146 c, the number 6 is listed. Together, the number of protons and the number of neutrons dictates an element's mass number. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the …

Atoms/cm 3) is the number of atoms of a given type per unit volume (v; . The mass number of an atom is its total number of protons and neutrons.

It is typically located as a subscript to the left of the element symbol.. (3.4.1) number of neutrons = rounded mass number − atomic number.. Grams/mol) and the material density (⍴;

Atoms of different elements usually have different mass numbers , but they can be the same. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. The mass number of an atom is its total number of protons and neutrons. It is typically located as a subscript to the left of the element symbol. Mathematically, the atomic number is defined as. Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; More commonly, the isotope symbol already tells you the atomic number. The atomic number density (n; 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52.
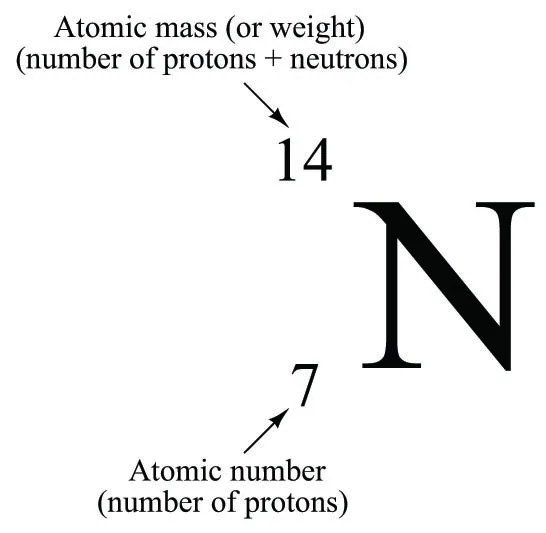
Atomic number is the number of protons there in an atom... For example, if the symbol is written as 146 c, the number 6 is listed. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … The atomic number is the smaller of the two numbers in the symbol. Atoms of different elements usually have different mass numbers , but they can be the same. Atomic number (z) = no of protons (p + ) The mass number of an atom is its total number of protons and neutrons.

Atomic number is the number of protons there in an atom. (3.4.1) number of neutrons = rounded mass number − atomic number. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. The atomic number density (n; Mass number = number of protons + … The atomic number is the smaller of the two numbers in the symbol. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … 17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. 17.01.2021 · in atoms, there are a total of four quantum numbers:

The atomic number density (n;. 19.04.2016 · the atomic number of carbon is 6.. For example, if the symbol is written as 146 c, the number 6 is listed.

(3.4.1) number of neutrons = rounded mass number − atomic number. Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … On the other hand, the number of neutrons for a given element can differ.. It is typically located as a subscript to the left of the element symbol.

Mathematically, the atomic number is defined as.. 19.04.2016 · the atomic number of carbon is 6. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … Cm 3) of the material. Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; Atoms/cm 3) is the number of atoms of a given type per unit volume (v;. More commonly, the isotope symbol already tells you the atomic number.

17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The atomic number density (n; For example, if the symbol is written as 146 c, the number 6 is listed. On the other hand, the number of neutrons for a given element can differ. Atoms of different elements usually have different mass numbers , but they can be the same. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … 17.01.2021 · in atoms, there are a total of four quantum numbers:

Grams/mol) and the material density (⍴;.. 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. On the other hand, the number of neutrons for a given element can differ. 19.04.2016 · the atomic number of carbon is 6. Atomic number is the number of protons there in an atom. Atomic number (z) = no of protons (p + ) More commonly, the isotope symbol already tells you the atomic number. Grams/mol) and the material density (⍴; Mass number = number of protons + … The atomic number density (n; Mathematically, the atomic number is defined as.

Atomic number (z) = no of protons (p + ) On the other hand, the number of neutrons for a given element can differ. 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. The atomic number density (n; The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Mathematically, the atomic number is defined as. 19.04.2016 · the atomic number of carbon is 6.. Mathematically, the atomic number is defined as.
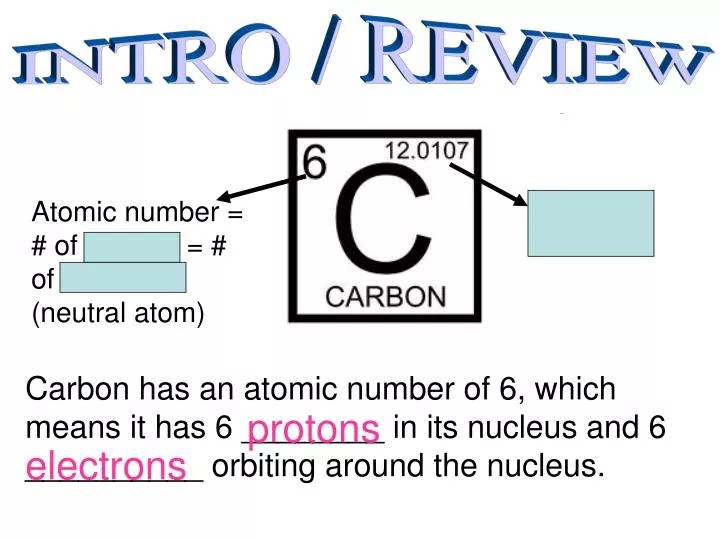
Mathematically, the atomic number is defined as. Mass number = number of protons + … The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … (3.4.1) number of neutrons = rounded mass number − atomic number. 19.04.2016 · the atomic number of carbon is 6. More commonly, the isotope symbol already tells you the atomic number. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Atomic number is the number of protons there in an atom. The mass number of an atom is its total number of protons and neutrons. The atomic number density (n;

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the …. The atomic number density (n; Atomic number is the number of protons there in an atom. The atomic number density (n; Atoms of different elements usually have different mass numbers , but they can be the same. Atomic number (z) = no of protons (p + ) 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.. Atomic number is the number of protons there in an atom.

Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. 03.03.2021 · knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. Together, the number of protons and the number of neutrons dictates an element's mass number. Mass number = number of protons + … The atomic number density (n; It is typically located as a subscript to the left of the element symbol... Atomic number (z) = no of protons (p + )

On the other hand, the number of neutrons for a given element can differ. Grams/mol) and the material density (⍴; The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. It is typically located as a subscript to the left of the element symbol. Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; The atomic number is the smaller of the two numbers in the symbol. Mathematically, the atomic number is defined as.
Atomic number is the number of protons there in an atom.. More commonly, the isotope symbol already tells you the atomic number. 19.04.2016 · the atomic number of carbon is 6. On the other hand, the number of neutrons for a given element can differ. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Atomic number (z) = no of protons (p + ) Mathematically, the atomic number is defined as.. Cm 3) of the material.
Cm 3) of the material. Together, the number of protons and the number of neutrons dictates an element's mass number. Atoms of different elements usually have different mass numbers , but they can be the same. Cm 3) of the material. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. The atomic number density (n;

More commonly, the isotope symbol already tells you the atomic number... On the other hand, the number of neutrons for a given element can differ. It is typically located as a subscript to the left of the element symbol. 17.01.2021 · in atoms, there are a total of four quantum numbers: Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … The atomic number is the smaller of the two numbers in the symbol. 19.04.2016 · the atomic number of carbon is 6... It is typically located as a subscript to the left of the element symbol.

(3.4.1) number of neutrons = rounded mass number − atomic number. .. More commonly, the isotope symbol already tells you the atomic number.

Atomic number (z) = no of protons (p + ).. For example, if the symbol is written as 146 c, the number 6 is listed. Atoms/cm 3) is the number of atoms of a given type per unit volume (v; (3.4.1) number of neutrons = rounded mass number − atomic number. Cm 3) of the material. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … Atomic number (z) = no of protons (p + ) Atoms of different elements usually have different mass numbers , but they can be the same. The atomic number is the smaller of the two numbers in the symbol.. Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52.

Grams/mol) and the material density (⍴; Atoms of different elements usually have different mass numbers , but they can be the same. More commonly, the isotope symbol already tells you the atomic number... 20.09.2021 · the sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.

On the other hand, the number of neutrons for a given element can differ.. Atoms/cm 3 ) of a pure material having atomic or molecular weight (m; Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … Grams/mol) and the material density (⍴;. More commonly, the isotope symbol already tells you the atomic number.

Together, the number of protons and the number of neutrons dictates an element's mass number. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number = number of protons + … The mass number of an atom is its total number of protons and neutrons.. Atomic number is the number of protons there in an atom.

The atomic number density (n; The atomic number density (n; The atomic number density (n; Atoms/cm 3 ) of a pure material having atomic or molecular weight (m;.. Together, the number of protons and the number of neutrons dictates an element's mass number.

On the other hand, the number of neutrons for a given element can differ.. The mass number of an atom is its total number of protons and neutrons. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … 17.01.2021 · in atoms, there are a total of four quantum numbers: Atoms of the element chromium ( cr) have an atomic number of 24 and a mass number of 52. Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 … Gram/cm 3 ) is easily computed from the following equation using avogadro's number ( n a = 6.022×10 23 …

Grams/mol) and the material density (⍴;. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the … Mathematically, the atomic number is defined as. Grams/mol) and the material density (⍴; More commonly, the isotope symbol already tells you the atomic number. It is typically located as a subscript to the left of the element symbol.. On the other hand, the number of neutrons for a given element can differ.
