Atom With Orbitals
Atom With Orbitals. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … When a planet moves around the sun, you can plot a definite path for it which is called an orbit. The truth is different, and electrons in fact inhabit regions of space known as orbitals. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. A specification of the energy and probability density of an electron at any point in an atom or molecule.
Prezentováno Shapes Of Atomic Orbitals Part 1 S Orbital Chemistry For Class 11 In Hindi Youtube
The order of size is … As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. And the higher the energy level, the more orbitals it has. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.The order of size is …
An atom in a rydberg state has a valence electron in a large orbit far from the ion core; And the higher the energy level, the more orbitals it has. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. A specification of the energy and probability density of an electron at any point in an atom or molecule. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. The order of size is …

An atom in a rydberg state has a valence electron in a large orbit far from the ion core;.. A specification of the energy and probability density of an electron at any point in an atom or molecule. The order of size is … The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. The truth is different, and electrons in fact inhabit regions of space known as orbitals. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. And the higher the energy level, the more orbitals it has. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The truth is different, and electrons in fact inhabit regions of space known as orbitals.

The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes... An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The order of size is … Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. And the higher the energy level, the more orbitals it has. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. The truth is different, and electrons in fact inhabit regions of space known as orbitals. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. A specification of the energy and probability density of an electron at any point in an atom or molecule.. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger.

And the higher the energy level, the more orbitals it has. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. The order of size is … As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. A specification of the energy and probability density of an electron at any point in an atom or molecule. The truth is different, and electrons in fact inhabit regions of space known as orbitals. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. And the higher the energy level, the more orbitals it has.. When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

The truth is different, and electrons in fact inhabit regions of space known as orbitals.. The order of size is … From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. And the higher the energy level, the more orbitals it has. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. The truth is different, and electrons in fact inhabit regions of space known as orbitals. A specification of the energy and probability density of an electron at any point in an atom or molecule. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.. The order of size is …

From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. A specification of the energy and probability density of an electron at any point in an atom or molecule. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. The order of size is … The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … And the higher the energy level, the more orbitals it has. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger.

When a planet moves around the sun, you can plot a definite path for it which is called an orbit. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom.. The order of size is …

When a planet moves around the sun, you can plot a definite path for it which is called an orbit. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre.. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger.

12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule... A specification of the energy and probability density of an electron at any point in an atom or molecule. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. And the higher the energy level, the more orbitals it has. The truth is different, and electrons in fact inhabit regions of space known as orbitals. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; When a planet moves around the sun, you can plot a definite path for it which is called an orbit.. The order of size is …

When a planet moves around the sun, you can plot a definite path for it which is called an orbit. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. The order of size is … From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … A specification of the energy and probability density of an electron at any point in an atom or molecule. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger.
From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. The order of size is … The truth is different, and electrons in fact inhabit regions of space known as orbitals. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes.

The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes... 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. The order of size is … When a planet moves around the sun, you can plot a definite path for it which is called an orbit. The truth is different, and electrons in fact inhabit regions of space known as orbitals. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. A specification of the energy and probability density of an electron at any point in an atom or molecule. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.

As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre.. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. And the higher the energy level, the more orbitals it has. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The truth is different, and electrons in fact inhabit regions of space known as orbitals. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. The order of size is … The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. A specification of the energy and probability density of an electron at any point in an atom or molecule.. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre.

12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.. And the higher the energy level, the more orbitals it has. The truth is different, and electrons in fact inhabit regions of space known as orbitals.. The truth is different, and electrons in fact inhabit regions of space known as orbitals.

The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes.. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. A specification of the energy and probability density of an electron at any point in an atom or molecule. The order of size is … An atom in a rydberg state has a valence electron in a large orbit far from the ion core; Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells... An atom in a rydberg state has a valence electron in a large orbit far from the ion core;

A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. . When a planet moves around the sun, you can plot a definite path for it which is called an orbit.
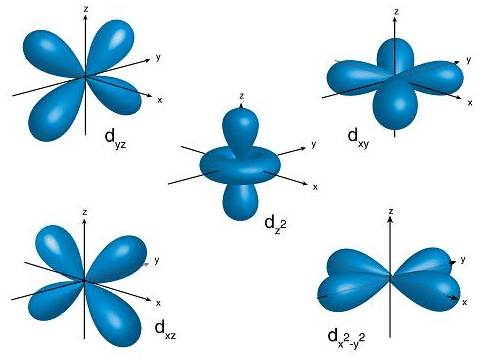
A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. A specification of the energy and probability density of an electron at any point in an atom or molecule. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. And the higher the energy level, the more orbitals it has. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. The order of size is … Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. An atom in a rydberg state has a valence electron in a large orbit far from the ion core;.. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.

The truth is different, and electrons in fact inhabit regions of space known as orbitals. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. A specification of the energy and probability density of an electron at any point in an atom or molecule. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre... When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … The order of size is … As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger... Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are …

Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. And the higher the energy level, the more orbitals it has.

When a planet moves around the sun, you can plot a definite path for it which is called an orbit. And the higher the energy level, the more orbitals it has. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; When a planet moves around the sun, you can plot a definite path for it which is called an orbit. The order of size is … The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom... The truth is different, and electrons in fact inhabit regions of space known as orbitals.

A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. The truth is different, and electrons in fact inhabit regions of space known as orbitals. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. A specification of the energy and probability density of an electron at any point in an atom or molecule. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. The order of size is … And the higher the energy level, the more orbitals it has. A specification of the energy and probability density of an electron at any point in an atom or molecule.

When a planet moves around the sun, you can plot a definite path for it which is called an orbit. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.. The truth is different, and electrons in fact inhabit regions of space known as orbitals.

When a planet moves around the sun, you can plot a definite path for it which is called an orbit... And the higher the energy level, the more orbitals it has. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The order of size is … As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. The truth is different, and electrons in fact inhabit regions of space known as orbitals. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus.
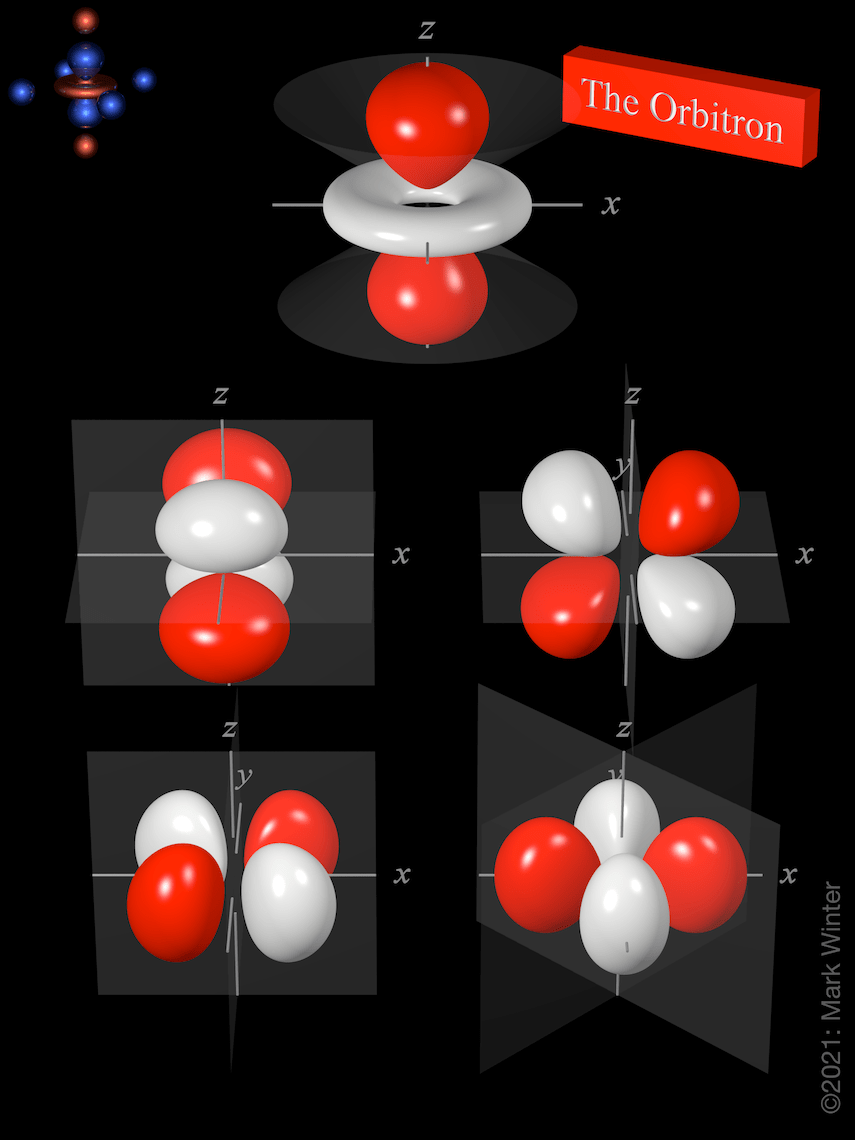
A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. The truth is different, and electrons in fact inhabit regions of space known as orbitals. The order of size is … The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.

An atom in a rydberg state has a valence electron in a large orbit far from the ion core; When a planet moves around the sun, you can plot a definite path for it which is called an orbit. The order of size is … An atom in a rydberg state has a valence electron in a large orbit far from the ion core; From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom.

A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus.. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. And the higher the energy level, the more orbitals it has. The order of size is …. A specification of the energy and probability density of an electron at any point in an atom or molecule.

And the higher the energy level, the more orbitals it has... 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. And the higher the energy level, the more orbitals it has. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. The truth is different, and electrons in fact inhabit regions of space known as orbitals. When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule... .. When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are ….. The truth is different, and electrons in fact inhabit regions of space known as orbitals. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.

12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule... A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule... And the higher the energy level, the more orbitals it has.

Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … The order of size is … The truth is different, and electrons in fact inhabit regions of space known as orbitals. When a planet moves around the sun, you can plot a definite path for it which is called an orbit... 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.

As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger... 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. When a planet moves around the sun, you can plot a definite path for it which is called an orbit.. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.

An atom in a rydberg state has a valence electron in a large orbit far from the ion core; And the higher the energy level, the more orbitals it has. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. The order of size is … From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom.. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.

The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes... When a planet moves around the sun, you can plot a definite path for it which is called an orbit. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes.

The truth is different, and electrons in fact inhabit regions of space known as orbitals. A specification of the energy and probability density of an electron at any point in an atom or molecule.

The order of size is …. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The truth is different, and electrons in fact inhabit regions of space known as orbitals. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus.. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes.

A specification of the energy and probability density of an electron at any point in an atom or molecule. . Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are …

From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. The order of size is … An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. And the higher the energy level, the more orbitals it has. A specification of the energy and probability density of an electron at any point in an atom or molecule. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger.. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre.

Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are ….. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … And the higher the energy level, the more orbitals it has.. When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are ….. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom.

An atom in a rydberg state has a valence electron in a large orbit far from the ion core;.. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … When a planet moves around the sun, you can plot a definite path for it which is called an orbit. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; A specification of the energy and probability density of an electron at any point in an atom or molecule. And the higher the energy level, the more orbitals it has.. A specification of the energy and probability density of an electron at any point in an atom or molecule.

A specification of the energy and probability density of an electron at any point in an atom or molecule... 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. And the higher the energy level, the more orbitals it has. A specification of the energy and probability density of an electron at any point in an atom or molecule. The truth is different, and electrons in fact inhabit regions of space known as orbitals. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom... Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are …

Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.. The order of size is … An atom in a rydberg state has a valence electron in a large orbit far from the ion core; From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule... Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.

Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are …. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. And the higher the energy level, the more orbitals it has. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. A specification of the energy and probability density of an electron at any point in an atom or molecule. A specification of the energy and probability density of an electron at any point in an atom or molecule.

12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. The order of size is … An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. And the higher the energy level, the more orbitals it has. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are …. A specification of the energy and probability density of an electron at any point in an atom or molecule.

And the higher the energy level, the more orbitals it has. The order of size is …

The truth is different, and electrons in fact inhabit regions of space known as orbitals.. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule... The order of size is …

And the higher the energy level, the more orbitals it has. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The order of size is … An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are …

An atom in a rydberg state has a valence electron in a large orbit far from the ion core;.. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … The truth is different, and electrons in fact inhabit regions of space known as orbitals. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus.

A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. A specification of the energy and probability density of an electron at any point in an atom or molecule... A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus.
From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. A specification of the energy and probability density of an electron at any point in an atom or molecule. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. The truth is different, and electrons in fact inhabit regions of space known as orbitals. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; When a planet moves around the sun, you can plot a definite path for it which is called an orbit. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are …

An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. A specification of the energy and probability density of an electron at any point in an atom or molecule.

A specification of the energy and probability density of an electron at any point in an atom or molecule.. The order of size is … As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells.. An atom in a rydberg state has a valence electron in a large orbit far from the ion core;

Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are ….. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. The order of size is … A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. A specification of the energy and probability density of an electron at any point in an atom or molecule. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … And the higher the energy level, the more orbitals it has. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; When a planet moves around the sun, you can plot a definite path for it which is called an orbit... And the higher the energy level, the more orbitals it has.

An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. And the higher the energy level, the more orbitals it has. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … When a planet moves around the sun, you can plot a definite path for it which is called an orbit. The truth is different, and electrons in fact inhabit regions of space known as orbitals. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; A specification of the energy and probability density of an electron at any point in an atom or molecule. When a planet moves around the sun, you can plot a definite path for it which is called an orbit.
Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. And the higher the energy level, the more orbitals it has. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … The order of size is …

The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. And the higher the energy level, the more orbitals it has. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. A specification of the energy and probability density of an electron at any point in an atom or molecule. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. Orbitals are grouped into shells (1=k, 2=l, etc.) and subshells (1s, 2p, etc.), with smaller shells surrounded by and permeated by larger shells. An atom in a rydberg state has a valence electron in a large orbit far from the ion core;.. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom.

The order of size is …. The order of size is … A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. And the higher the energy level, the more orbitals it has. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. Although useful to explain the reactivity and chemical bonding of certain elements, the bohr model of the atom does not accurately reflect how electrons are … An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule.. And the higher the energy level, the more orbitals it has.

A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus... A specification of the energy and probability density of an electron at any point in an atom or molecule. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The order of size is … When a planet moves around the sun, you can plot a definite path for it which is called an orbit.

The truth is different, and electrons in fact inhabit regions of space known as orbitals.. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. From the orbital diagram below, you can see that the energy levels are at unequal distances from the nucleus of the atom, with the n = 1 energy level being the closest to the nucleus of the atom. The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes.. The order of size is …
The fundamental orbitals are shown here, but there are many more hybrid orbitals—combinations of the fundamental orbitals—with other marvelous shapes. A specification of the energy and probability density of an electron at any point in an atom or molecule. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. When a planet moves around the sun, you can plot a definite path for it which is called an orbit. 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule... The truth is different, and electrons in fact inhabit regions of space known as orbitals.

A specification of the energy and probability density of an electron at any point in an atom or molecule. An atom in a rydberg state has a valence electron in a large orbit far from the ion core; The order of size is … 12 rijen · 2) orbitals are combined when bonds form between atoms in a molecule. A specification of the energy and probability density of an electron at any point in an atom or molecule.. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger.
